About Us
PQE Group is an ISO 9001 certified technology solutions and compliance consulting services company with global capabilities deliverable throughout the entire product quality life cycle.
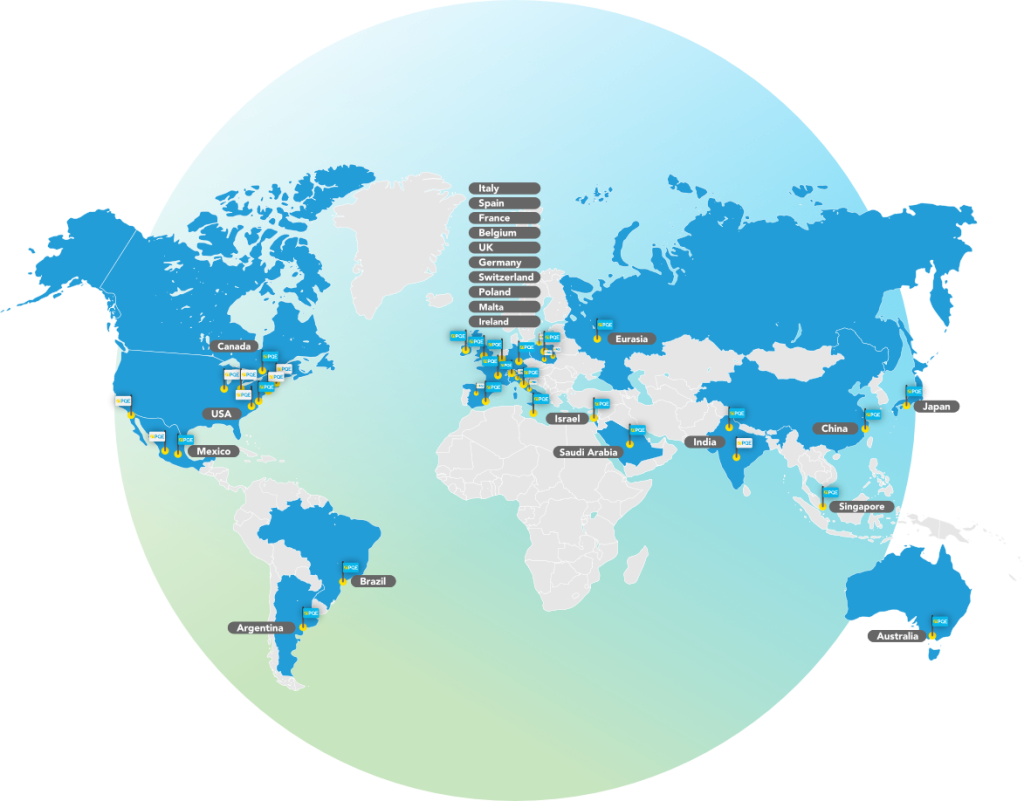
With offices located worldwide and a full-ranged broad service portfolio, extensive experience, effective project management, and exceptional cost effectiveness, PQE’s solutions are proven to be the winning combination for global corporations, as well as small and medium sized companies.
What makes PQE what it is?
Our History
We started off as a small start up in Tuscany, Italy, made of 5 Senior Consultants with a common interest: delivering the highest expertise within the Lifesciences space and ensure patients’ safety.
It's all about the people
We count on each person that works with us, as we grow stronger together.
Achieving our vision
Our vision is to create a diverse work environment with no discrimination and where transparency and respect come first. This is our strength.
An Award-Winning company

Best Managed
Company
2021 | 2022 | 2023
Deloitte Private

Top Champion Company four years in a row
2018 | 2019 | 2020 | 2021
Italy Post

Among the 1000 Fastest Growing Companies in Europe
2018
Financial Times

Nominated one of the 1000 companies to Inspire Europe in 2018
London Stock Exchange
Global Board of Directors






The PQE Federation: a global network of alliances
PQE Group has an established network of collaborations and alliances all over the world to complement services’ delivery, enhance capabilites’ portflio and scale up projects faster and efficiently.
Our M&A process over the years has been developed in a Federation Model and includes the following brands:



Corporate Social Responsibility
We strongly believe that our success is tied to the well-being of the community and the environment which we belong to.
Making ours Ghandi’s “Be the Change you wish to see in the World” motto, we take it into action with our commitment to Social Responsibility goals like Sustainable Development, Gender Equality, protection of the Environment and Education.



